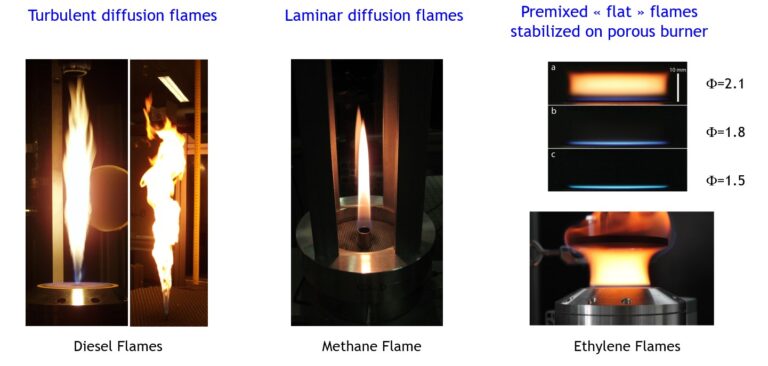
Credits : France SIMONET/IRCELYON/CNRS Photothèque
Topic 2 :
CHEMICAL ANALYSIS OF THE PARTICULATE AND/OR GASEOUS PHASES ADSORBED ON OR ABSORBED BY SOOT
There are different levels of characterisation of the chemical composition of soot particles. Thermo-optical elemental carbon/organic carbon speciation allows the characterisation of soot particles as a whole, quantifying the share of each of these two fractions, but without providing information at the molecular level. On the other hand, molecular analysis techniques, both inorganic and organic, allow access to the detailed composition of the particles.
Knowing their composition to understand their reactivity
It can include :
- elemental carbon, which is defined as “Black Carbon (BC)”, when optically characterised,
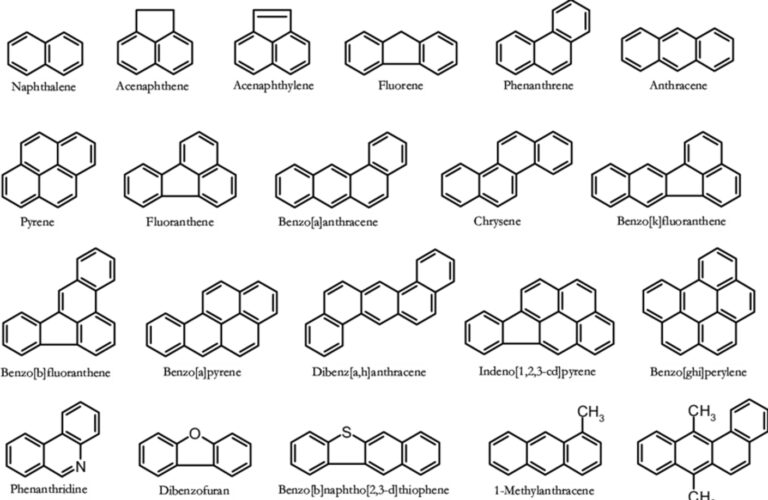
- an organic fraction made up of a large number of molecules of intermediate or low volatility such as alkanes or hopanes as well as PAHs which, with their oxygenated and nitrated derivatives, constitute one of the most important families
- a multitude of metals ranging from Al to Rb, including Ti and Si,
- inorganic nitrogenous and sulphurous species such as HNO3 and H2SO4 acids.
In the atmosphere and in the flue gases/pyrolysis within industrial processes, soot particles are emitted and transported in the simultaneous presence of volatile and semi-volatile organic compounds as well as acid gases and metallic elements. The soot particles themselves consist of a semi-volatile fraction and are thus in permanent interaction with the gas phase through condensation/evaporation processes directly influenced by temperature and pressure variations, flow regimes, dilution of the air masses considered and by reaction averages, in particular with the oxidants present in the gas phase.
It is therefore essential when characterising the chemistry of soot particles to study both their surface chemical composition, in addition to their composition considered in their volume, and the composition of the associated gas phase.
In addition, it is important to emphasise that, in addition to the chemical complexity of soot, its composition is subject to significant variability at the source due to the diversity of the fuels used (wood, coal, paraffin, etc.), combustion conditions (temperature, richness, etc.) and also their evolution (see theme 3: soot ageing). Thus, the search for specific molecular tracers (e.g. levoglucosan for wood combustion) and isotopes is an essential tool for assessing the contribution of soot particle sources in the atmosphere.